We just made a couple fixtures and it's gonna help us run these parts for autodesk non-stop where you can run 20 parts a hundred parts out of time and just clips and it just keeps going I have to figure out how to run the parts as efficiently as possible and make it cheaper than somebody else so each one slides in then we take this mighty bite pit bull clamp and I've popped this guy right in on the inside the second up what we have here is we have another clamp this guy actually drops down and as the bolt goes straight down it spreads locking one part here one part here it's gonna rotate and it will just keep doing the same thing over and over instead of a part taking six minutes it takes 50 seconds therefore I can make good money I can pay my employees and just keep the ball rolling.
PDF editing your way
Complete or edit your property disclosure statement anytime and from any device using our web, desktop, and mobile apps. Create custom documents by adding smart fillable fields.
Native cloud integration
Work smarter and export ownership disclosure statement directly to your preferred cloud. Get everything you need to store, synchronize and share safely with the recipients.
All-in-one PDF converter
Convert and save your disclosure ownership statement as PDF (.pdf), presentation (.pptx), image (.jpeg), spreadsheet (.xlsx) or document (.docx). Transform it to the fillable template for one-click reusing.
Faster real-time collaboration
Invite your teammates to work with you in a single secure workspace. Manage complex workflows and remove blockers to collaborate more efficiently.
Well-organized document storage
Generate as many documents and template folders as you need. Add custom tags to your files and records for faster organization and easier access.
Strengthen security and compliance
Add an extra layer of protection to your disclosure ownership interest statement by requiring a signer to enter a password or authenticate their identity via text messages or phone calls.
Company logo & branding
Brand your communication and make your emails recognizable by adding your company’s logo. Generate error-free forms that create a more professional feel for your business.
Multiple export options
Share your files securely by selecting the method of your choice: send by email, SMS, fax, USPS, or create a link to a fillable form. Set up notifications and reminders.
Customizable eSignature workflows
Build and scale eSignature workflows with clicks, not code. Benefit from intuitive experience with role-based signing orders, built-in payments, and detailed audit trail.
Award-winning PDF software





How to prepare HCFA-1513
About HCFA-1513
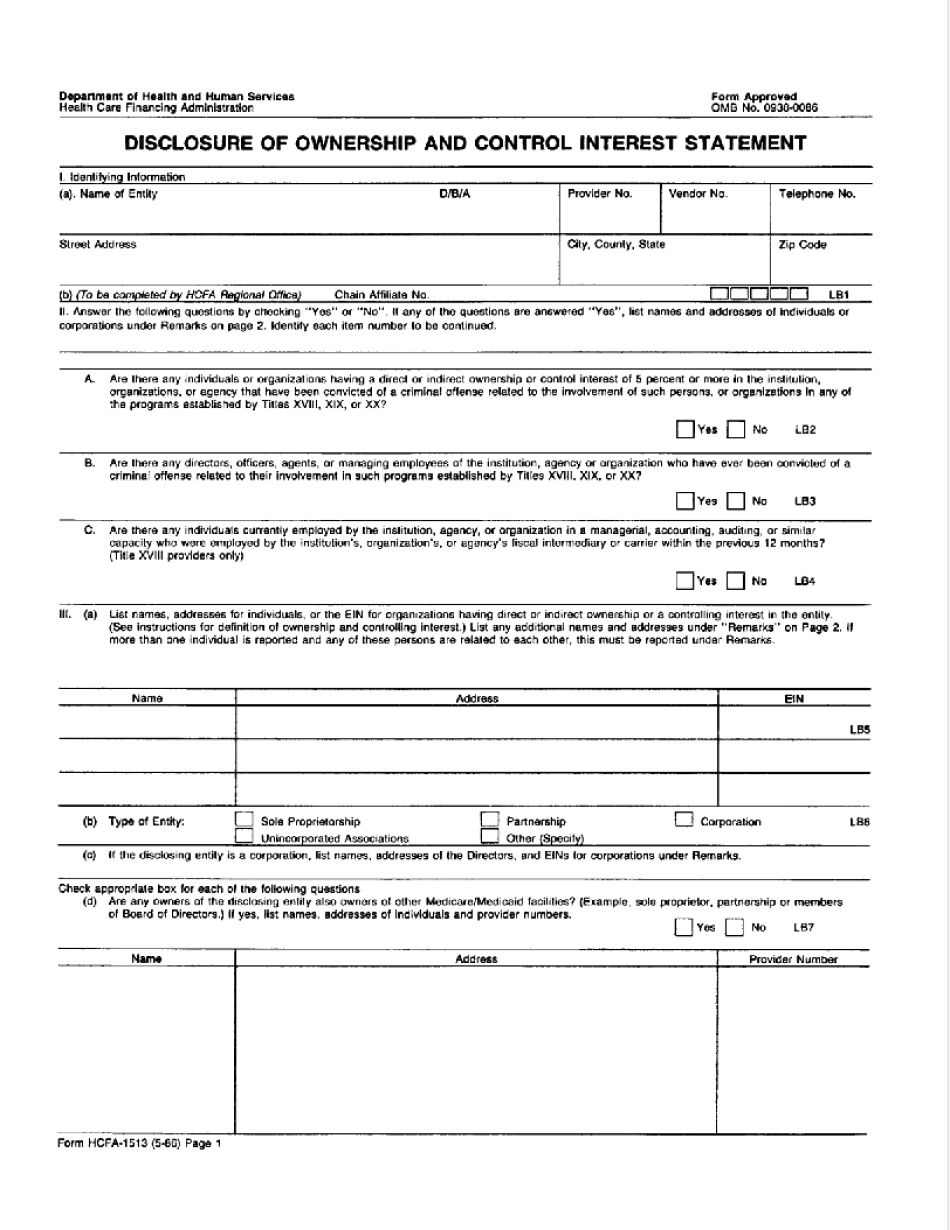
HCFA-1513 refers to the Health Care Financing Administration (HCFA) form 1513. It is a document used to report outpatient rehabilitation therapy services provided to Medicare beneficiaries. This form is specifically designed for physical therapy, occupational therapy, and speech-language pathology services. Medicare beneficiaries who receive outpatient rehabilitation therapy services, such as physical therapy, occupational therapy, or speech-language pathology services, are required to have their providers use the HCFA-1513 form. This form allows for accurate reporting and billing of these therapeutic services to Medicare. It contains information about the patient, provider, dates of service, therapy codes, charges, and other relevant details. Essentially, anyone who receives outpatient therapy services covered by Medicare would require their providers to complete HCFA-1513 forms. These forms help ensure proper documentation, billing, and reimbursement for the therapy services provided to Medicare beneficiaries.
Online solutions assist you to organize your document management and strengthen the productivity of your workflow. Follow the brief manual to be able to complete HCFA-1513, stay away from mistakes and furnish it in a timely manner:
How to fill out a Disclosure Of Ownership And Control Interest Statement?
-
On the website containing the blank, click Start Now and go for the editor.
-
Use the clues to fill out the relevant fields.
-
Include your personal data and contact information.
-
Make absolutely sure you enter proper details and numbers in appropriate fields.
-
Carefully review the written content in the blank as well as grammar and spelling.
-
Refer to Help section if you have any questions or address our Support team.
-
Put an electronic signature on your HCFA-1513 printable while using the assistance of Sign Tool.
-
Once document is done, click Done.
-
Distribute the prepared document through email or fax, print it out or download on your device.
PDF editor enables you to make modifications to your HCFA-1513 Fill Online from any internet connected device, customize it according to your needs, sign it electronically and distribute in several means.
What people say about us
File documents in a timely manner with a trustworthy online tool
Video instructions and help with filling out and completing HCFA-1513